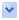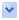A risk-benefit analysis of gene editing tools in stem cells revealed that base and prime editing carry vulnerabilities similar to those of CRISPR-Cas9, but at a reduced rate.
基因編輯工具,於幹細胞中之風險與效益的分析,揭露了鹼基及引子(prime)編輯具有,與CRISPR-Cas9(CRISPR:Clustered Regularly Interspaced Short Palindromic Repeat:成簇、規律性間隔開的短迴文結構複製)-( Cas9:CRISPR associated protein 9:CRISPR相關的蛋白質9)類似的脆弱性。不過,處於縮小的比率狀態。

圖1. 切割DNA之一股或兩股的基因編輯工具,會將非想要的突變引入基因體內。
Gene editing tools that cut one or both strands of DNA can introduce undesired mutations into the genome.
New gene editing tools could become gamechangers for gene therapy, but scientists need to develop new approaches that curtail undesirable mutations. The clustered regularly interspaced short palindromic repeats (CRISPR-Cas9 system is one such error-prone editing tool) that scientists later modified to limit its mutational burden.
就基因療法而言,新的基因編輯工具,可能成為編輯規則的改變者。不過,科學家們需要開發,減少不良突變的新方法。這種成簇規則性間隔開的短回文結構複製(CRISPR-Cas9方法是一種,這樣容易出錯的編輯工具),後來經科學家們修改,來限制其突變負荷。
However, it’s unclear how the adapted techniques, called base and prime editing, stack up against the original CRISPR-Cas9 system. Reporting in the journal Nature Biotechnology, researchers at the San Raffaele Scientific Institute revealed that base and prime editing produced more mutations than previously suggested but produced them less often than CRISPR-Cas9.
然而,這種被稱為鹼基及引子編輯的改編技術,如何能匹敵原本的CRISPR-Cas9方法,目前不詳。於《自然•生物技術》期刊的報告中,義大利聖拉斐爾科學研究所的研究人員們揭露了,鹼基及引子編輯產生了,多於先前被提出的突變。不過,比CRISPR-Cas9較少頻繁產生此些突變。
CRISPR-Cas9 gene editing relies on a guide RNA that binds to a desired DNA sequence and a Cas9 enzyme that cuts both strands of DNA at that site, creating a double-strand break. Scientists edit the sequences at the cut ends before using different approaches to guide repair.
CRISPR-Cas9基因編輯仰賴,與渴望之DNA序列結合的導引RNA,及在那產生雙股斷裂位點,切割DNA之兩股的Cas9酶。科學家們,在使用不同的方法來引領修復之前,編輯於切割端的序列。
However, there are often additional DNA sequences with blunt ends in the nucleus that could accidentally get jumbled with the cut ends during repair, leading to mutations. Therefore, researchers adapted CRISPR-Cas9 techniques to nick a base out of one strand while leaving the complementary strand intact, thereby preventing the random joining of blunt and cut ends that lead to mutations.
然而,細胞核中經常存在具有平末端(指DNA重組技術中,DNA限制酶在切開DNA的雙股結構時,形成突出末端與平末端。平末端則是上述切割過程中不突出的末端)的額外DNA列。這些序列在修復過程中,可能意外地與切割端混淆,而導致突變。因此,研究人員們採用了,CRISPR-Cas9技術,來從一股上切掉一個鹼基,同時保持互補股完整。從而防止,導致突變之平末端與切割端的任意連接。
Luigi Naldini, a gene therapy researcher and coauthor of the study, has run cost-benefit analyses of gene editing tools throughout his career. He said, “It was obvious to us that we [should] similarly investigate whether base editing indeed provided a benefit over the previous editing technology.”
該項研究合撰人,基因療法研究員Luigi Naldini,在其整個職業生涯中,一直進行基因編輯工具的成本效益分析。他宣稱:「對我們而言,顯然我們同樣[應該]調查研究,鹼基編輯是否確實提供,超越先前之編輯技術的益處。」
He hypothesized that when the nicked strand unzips from the intact complementary strand during replication, it could serve as a template to make a second nicked strand. This would create a DNA double helix with a blunt end that could randomly join up with another blunt end, thus rendering the DNA vulnerable to mutations.
他假設,在複製過程中,當經切割之股,從完整的互補股上解開時,它可能充當一種模板,來製造第二條切割的股。這會產生一條,具有可任意與另一平末端連接之平末端的DNA雙螺旋。因此,使得該DNA易有突變。
To find out whether base editors were truly less error-prone than CRISPR-Cas9 tools, the team compared their DNA editing performance. They chose to target the gene for β2-microglobulin because it encodes a cell-surface protein that is easy to detect and quantify with antibodies.
為了查明鹼基編輯器,是否真的比CRISPR-Cas9工具較不易出差錯。該團隊比較了,它們的DNA編輯性能。他們選擇了鎖定,有關β2-微球蛋白的基因。因為它編碼一種,容易使用抗體,來檢測及量化的細胞表面蛋白質。
Using both technologies, they modified the gene in hematopoietic stem cells (HSC), a common gene therapy target for blood disorders. Both tools deleted the gene with comparably high efficiency (approximately 80 to 90 percent), but base editors produced far fewer undesired mutations than the CRISPR-Cas9 tool.
他們使用這兩種技術修改了,造血幹細胞(HSC)中,一種就血液疾病而言,是常見基因療法標靶的基因。這兩種工具以可匹敵(大約80%到90%)的高效率,剔除了該基因。不過,鹼基編輯器產生了,遠少於CRISPR-Cas9工具,產生的不良突變。
As Naldini suspected, the team detected mutations arising from double-strand breaks in base edited cells, albeit at a lower level than in the CRISPR-Cas9 edited cells. These mutations occurred at the nicked site, suggesting that it was transformed into an accidental double-strand break during replication. “It’s wrong to say that this technology is break-free,” Naldini said.
如同Naldini懷疑的,該團隊在鹼基編輯之細胞中,發現了起源於雙股斷裂的突變,縱然處於比CRISPR-Cas9編輯之細胞中,較低的水平。此些突變存在於切割位點,暗示在複製過程中,這被轉化為意外的雙股斷裂。Naldini宣稱:「宣稱此技術免於斷裂,這是錯誤的。」
The team also explored whether the editing systems triggered undesired cellular responses. The CRISPR-Cas9 tool appeared to activate a DNA repair pathway governed by p53, a protein known as the “guardian of the genome.”
該團隊也探索了,此些編輯方法是否引發了,不良的細胞反應。CRISPR-Cas9工具似乎活化了,由p53控制的DNA修復途徑。p53是一種,被稱為“基因體守護者”的蛋白質。
This was expected for an editing tool that deliberately generates double-strand breaks; however, they also detected p53 activation at a reduced level for a specific base editor that converts cytosine into thymine.
就故意產生雙股斷裂的編輯工具而言,這是被預期的。然而,他們也發現,有關一種將胞嘧啶轉化成胸腺嘧啶之特定鹼基編輯器的p53活化作用,是處於降低水平的狀態。
CRISPR-Cas9 outperformed base editors in one regard: It didn’t trigger innate immunity. Base editors use long guide RNAs that are viewed by the cell as foreign, triggering immune responses.
在某一點上,CRISPR-Cas9性能優於鹼基編輯器:它沒有觸發先天免疫力。鹼基編輯器使用,被細胞視為,觸發免疫反應之外來物的長導引RNA。
These responses cause concern because the yield of edited HSC produced for gene therapy is never 100 percent. Over time, edited cells may die if they are outnumbered by unedited ones unencumbered by stress responses, reducing the durability of the therapy.
此些反應引起了憂慮。因為,供作基因療法使用、經編輯之造血幹細胞的產量,不曾是100%。隨著時間推移,倘若經編輯之細胞數量,被未經編輯的細胞超越,且不受壓力反應影響,則它們可能死亡,而降低此療法的持久性。
“It’s probably negative selection of those cells that experienced maybe the highest burden of detrimental responses and outcomes,” suggested Samuele Ferrari, a biotechnologist and coauthor of the study.
該項研究合撰人,生物科技學家Samuele Ferrari委婉表示:「這可能是,那些經歷了或許是有害反應及結果,最高負擔之細胞的消極選擇。」
“Probably the main message is the safety of the novel technology,” said Annarita Miccio, a molecular biologist at the Institut Imagine who was not involved with the work.
法國遺傳疾病想像研究所,未涉及該項研究的分子生物學家,Annarita Miccio宣稱:「主要訊息可能是,該項新技術的安全性。」
Her colleague Mégane Brusson, who also was not involved in the study, added, “It’s true that there are less genotoxic risks with base editors and prime editors, but as they show in this paper, there is still some concern that we have to take into account when we develop gene therapy or genome-editing strategies for therapeutic approaches.”
她也未涉及該項研究的同僚,Mégane Brusson附言:「確實,使用鹼基及引子編輯器,有較少的基因毒性風險。不過,如同他們於此文中指出的,當我們開發基因療法或基因體編輯策略,作為為治療方法時。仍然有一些,我們必須納入顧慮之事。」
Fortunately, the researchers found a way to reduce most of the genotoxic effects of base editors to an undetectable level by first optimizing the guide RNAs and then lowering the dosage used.
幸運的是,此些研究人員找到了一種,首先藉由最佳化導引RNAs,然後降低使用劑量,來使鹼基編輯器之大部分基因毒性效應,降低到不可檢測水平的方法。
To prolong the lifespan of RNA, they added features like a 5-prime cap that prevent their breakdown in the cell. However, optimization did not completely abolish all undesired effects. Base editors still triggered mutations at random sites in the genome beyond the target site.
為了延長RNA的壽命,他們添加了,諸如防止它們在細胞中分解之5端帽(是在真核生物中信使RNA的5′端經修改後形成的雙核苷酸端點)等的諸多特點。然而,最佳化並未完全消除所有不良影響。在標的位點之外,鹼基編輯器仍然於基因體中的任意位點,引發了突變。
Lastly, the team explored prime editing, the other nicking technique. Instead of modifying a single base pair, it removes several bases out of one strand of a gene and inserts new ones in their place. “This is the latest kid on the block among all the editing tools,” Ferrari said, adding that owing to its infancy, “Prime editing has not been yet tested in clinical settings.”
最後,該團隊探索了引子編輯,另一種切割技術。代替修改單一鹼基對,它從基因的一股中,移除幾個鹼基,並在其位置嵌入新的鹼基。Ferrari宣稱:「在所有編輯工具這區塊中,這是最新的工具。」補充說,由於其起步階段。「引子編輯尚未在臨床環境中,進行測試。」
Similar to base editing, prime editing led to mutations at the nick site that signified the accidental formation of double-strand breaks and triggered the p53 DNA repair pathway plus immune signaling. As with base editing, the tool will need to be optimized to avoid these unwanted cellular responses.
與鹼基編輯類似,引子編輯也導致了,於切割位點,意味著雙股斷裂意外形成,及除了免疫發信號外,也觸發了p53 DNA修復途徑的突變。如同使用鹼基編輯,該工具將需被最佳化,以避免此些不需要的細胞反應。
Base and prime editing also spawn undesired effects that can only be spotted with scrupulous screening. “As a field, we don’t know too much about data in clinical trials regarding, for example, long-range deletions,” noted Ferrari.
鹼基及引子編輯也引發,只能使用嚴格篩選,才能被發現的不良影響。Ferrari特別提及:「作為一個領域,有關臨床試驗中的數據,太多我們不知道。譬如,長範圍的缺失。」
Brusson said, “In the future, we need more powerful technology to better characterize the genotoxic effects of base and prime editing technology.”
Brusson宣稱:「於未來,我們需要更強有力的技術,來更佳地表徵,鹼基及引子編輯技術的基因毒性作用。」
Miccio and her team are exploring epigenome editing as an alternative tool for modulating genes in therapy. These tools activate or repress genes by modifying chromatin structure, and they don’t introduce mutations in the genome. “This would theoretically be the best and safest strategy that would definitely avoid all these genotoxic effects because there is no enzyme that is modifying the DNA,” she said.
Miccio及其團隊正進行探索,漸成論(表觀)基因體編輯,作為在治療中,供調節基因之用的替代工具。此些工具藉由修改染色質結構,來活化或抑制基因。因此,不會於基因體中,引入突變。她宣稱:「理論上,這會是肯定能避免,所有此些基因毒性作用之最佳、最安全的策略。因為,沒有正在修飾DNA的酶。」
UK regulators recently approved CRISPR-Cas9 for treating sickle-cell disease and β-thalassemia, and researchers have already used base-edited cells to treat leukemia in a 13-year old girl in the UK . Although these editing tools have the potential to save lives, this study suggests that it may be worth further examining their genotoxic risks.
最近,英國監管機構批准CRISPR-Cas9,供治療鐮狀細胞疾病及β地中海型貧血之用,且研究人員們已經使用鹼基編輯細胞,來治療英國一名13歲女孩的白血病。雖然,些編輯工具有拯救生命的潛力。不過,該項研究暗示,這或許值得,進一步探究它們的基因毒性風險。
網址:https://www.the-scientist.com/news/genotoxic-effects-of-base-and-prime-editing-71587
翻譯:許東榮
文章定位: