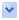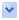Combining two cutting-edge technologies, researchers revealed the impact of a multitude of genes that are associated with neurodevelopmental disorders, including autism, but whose effects on human brain development were previously unknown.
結合兩項尖端技術,研究人員們揭露了,多種與神經發育障礙(包括自閉症)相關基因的影響。不過,那些對人類大腦發育的影響,先前是不詳的。

圖1. 前腦組合體:人類遷移之中間神經元為綠色;細胞核呈粉紅色。(圖援用自原文)
Forebrain assembloids: The human migrating interneurons are in green; nuclei are in pink.
Stanford Medicine investigators and their colleagues sifted through a jumble of genes implicated in neurodevelopmental disorders and identified dozens of disparate troublemakers with similar effects.
美國史丹佛大學醫學院的調查研究人員及其同事們探究了,一堆涉及神經發育障礙的基因,且發現了數十種,具有相似影響之本質上不同的惹事生非者。
Because the method they used sorts defective genes by their function — or in this case, their dysfunction — the approach is likely to accelerate drug development for neurodevelopmental disorders.
因為他們使用的方法,根據基因功能(也就是,在此實例中,是其功能障礙) 分類有缺陷的基因。因此該方法可能加速,有關神經發育障礙的藥物開發。
Studies have implicated at least 500 genes in such disorders. But scientists have no idea exactly how defects in most of these genes impair brain function.
諸多研究已經顯示,在此類障礙中,至少500個基因涉及。不過,在大多數此些基因中的缺陷,確切如何損傷大腦功能,科學家們並不無概念。
The research, which points the way toward creating order from this chaos, is described in a paper published online Sept. 27 in Nature. Sergiu Pasca, MD, the Kenneth T. Norris, Jr. Professor II of Psychiatry and Behavioral Sciences, is the study’s senior author. The lead author is Xiangling Meng, PhD, a postdoctoral scholar in Pasca’s group.
在2023年9月27日,發表於網路版《自然》期刊的一篇論文中,描述了該項將方法指向,從這種混亂中創造秩序的研究。精神病學暨行為科學教授,Sergiu Pasca醫學博士是該項研究的資深撰文人。首要撰文人,是史丹佛大學Sergiu Pasca教授團隊中的博士後學者,Xiangling Meng博士。
There are two main classes of neurons in the cerebral cortex: excitatory and inhibitory. Excitatory neurons fire impulses that activate other neurons, while inhibitory neurons’ firing blocks other neurons from firing. Inhibitory and excitatory neurons integrate to form circuits, shaping signaling activity in the brain.
在大腦皮質中,有刺激性及抑制性兩類主要神經元。刺激性神經元發射,激活其他神經元的脈衝,而抑制性神經元的發射,阻擾其他神經元發射。抑制性及刺激性神經元整合形成,於大腦中形成發信號活動的網路。
In humans, as many as half of all cells in the cerebral cortex, the brain’s outermost and most recently evolved layer, are inhibitory. Scientists theorize that an imbalance in the number or function of inhibitory neurons compared with excitatory neurons might be at least partly responsible for autism spectrum disorder and epilepsy.
在人類中,於大腦最外層且是最新近演化的大腦皮質中,多達一半細胞是抑制性的。科學家們理論推測,與刺激性神經元相較下,在抑制性神經元的數量或功能上的不平衡,至少可能是導致自閉症系列障礙及癲癇的部分原因。
“If that’s true, you could find ways to alter the functional balance of these cells in the cortex as a therapeutic approach for these disorders,” Pasca said. But first, he wondered, how do you make sense of the enormous collection of genes that have been implicated in these conditions but whose impacts are largely unknown?
Pasca宣稱:「倘若那是真的,那麼可能找到方法,來改變皮質中,此些細胞的功能平衡,作為此些疾病的一種治療方法。」不過首先,他極想知道,如何理解已經涉及此些疾病,不過其影響絕大部分不詳的巨大量基因?
Does the existence of hundreds of genes associated with disease mean that there are hundreds of different types of neurodevelopmental disorders, each requiring a different remedy? Or might several different genes converge and lead to similar pathology, or some particular aspect of it?
數百個與疾病相關基因的存在,意味著有數百種不同類型之神經發育障礙,每種皆需要一種不同的治療法?或可能幾個不同的基因聚合,而導致相似的病變,或其某些特定層面?
“If it’s the latter, a cluster of gene defects that all produce a similar physiological deficit might be amenable to a single type of treatment,” Pasca said.
Pasca宣稱:「倘若是後者,則所有產生相似生理缺陷的一群基因缺陷,可能經得起單一類型的治療。」
Until recently, there was no way to study early brain development in a human being. But Pasca, who is also the Bonnie Uytengsu and Family Director of the Stanford Brain Organogenesis Program, pioneered a technology that makes detailed exploration of the developing human brain possible.
直到最近,一直沒於人類中,研究初期的大腦發育。不過,也是史丹佛大學腦器官發生計劃之Bonnie Uytengsu及家族主任的Pasca開創了一項,使詳細探索發育中之人類大腦,成為可能的技術。
With special laboratory glassware; a combination of growth factors (substances that stimulate cell growth) and nutrients; and human induced pluripotent stem cells — or iPS cells, which can be generated from a simple skin biopsy — he can generate small clumps of neural tissue whose anatomical architecture and function closely resemble part of the brain, such as the human cerebral cortex. Pasca calls these clumps cortical organoids.
使用特殊的實驗室玻璃器皿、生長因子(刺激細胞生長的物質)與營養素的組合及人類誘導的多能幹細胞(也就是,能從簡單的皮膚切片被產生的iPS 細胞)。他能產生諸多,解剖結構及功能與部分大腦非常相似的小塊神經組織,諸如人類大腦皮質。Pasca稱此些小塊為皮質類器官。
Adjusting the nutrients and growth factors in which iPS cells are bathed, Pasca has shown, generates organoids approximating another brain structure called the subpallium. Located deeper in the forebrain than the cortex, the subpallium plays a key role during fetal and infantile development:
調整iPS細胞被浸泡其中的營養素及生長因子,Pasca已經證實,產生近乎另一種,被稱為大腦皮質下層的類器官。位於前腦中比皮質更深處的大腦皮質下層,在胎兒及嬰兒發育過程中,扮演一種關鍵角色:
It produces inhibitory neurons, known as interneurons, that migrate to the cortex and elsewhere and join up with excitatory neurons to form functioning circuits capable of complex signal generation.
它產生,遷移到皮質及其他地方,並與刺激性神經元結合,形成能夠產生複雜訊號之功能電路,被通稱為中間神經元的抑制性神經元。
By placing subpallial organoids next to cortical organoids and waiting for several days, Pasca was able to observe, for the first time, how these two structures fuse together to form what he calls “assembloids” and to watch interneurons migrate from the subpallium to the cortex and hook up with excitatory neurons there.
藉由將大腦皮質下層的類器官,放置於皮質類器官旁邊,並等待幾天。Pasca首次能觀察到,這兩種結構如何融合在一起,形成他所稱呼的“組合體”,並觀察中間神經元,從該大腦皮質下層遷移到皮質,而與那裡的刺激性神經元接合起來。
In the new study, Pasca and his colleagues paired this cutting-edge technology with another: CRISPR. CRISPR employs a molecular scissors and a molecular bloodhound. This enables researchers to snip out specific DNA sequences at will, so they can knock selected genes out of the genome and see what happens.
在該項新研究中,Pasca及其同僚們結合了,該項尖端技術與群聚、規律性間隔開的短迴文結構複製(CRISPR:Clustered Regularly Interspaced Short Palindromic Repeat)的另一種技術。CRISPR使用一種分子剪刀及一種分子獵犬。這使研究人員們能隨意剪掉特定的DNA序列,因此他們能從基因體中,剔除選定的基因,來查明發生什麼。
Pasca and his colleagues narrowed the neurodevelopmental-disorder-associated genes on their list down to 425 that are activated in interneurons. They created iPS cells that when differentiated into multi-celled organoids fluoresced only in those cells that had become interneurons.
Pasca及其同僚們將他們清單中,與神經發育障礙相關的基因,縮減到425 個於中間神經元中,被活化的基因。他們創造了,當分化成多細胞的類器官時,僅在那些已經成為中間神經元之細胞中,發出螢光的iPS細胞。
This way, they could generate subpallial organoids in which they’d be able to distinguish interneurons from all the other brain-cell types the subpallium produces.
此方法,能產生於其中,他們能從大腦皮質下層產生的所有其他腦細胞類型,區別出中間神經元之大腦皮質下層的類器官。
They infected thousands of these cells — which had also been bioengineered to carry a gene whose protein product was the molecular scissors of CRISPR — with CRISPR “bloodhounds”:
使用CRISPR “獵犬”,他們感染了數千個,也已經被生物工程改造,來攜帶一種其產生的蛋白質,是CRISPR剪刀之基因的細胞:
small strips of nucleic acids that are complementary to portions of one or another of the 425 neurodevelopmental-disorder-associated genes of interest. Wherever such a strip affixed itself to a gene, the CRISPR “scissors,” an enzyme, would excise the DNA bound by the strip.
核酸的小長條,與令人感興趣之425個神經發育障礙相關基因中,一個或另一個的部分是互補的。無論在哪裡,這樣的長條自行固著於一個基因上,CRISPR“剪刀”(一種酵素)會切除被此長條結合的DNA。
Tossing enough of these bloodhounds into a large enough pool of these cells made it statistically likely that each of the 425 suspect genes would get snipped out in at least hundreds of cells in the pool.
將足夠的此些獵犬投入夠大的此些細胞池中,使其統計上,425個可疑基因的每一個,在此池至少數百個細胞中,可能會被剪掉。
From those iPS cells, the researchers generated subpallial organoids. Some showed no fluorescence at all after 44 days in a dish, indicating their failure to generate interneurons. By sequencing the genomes of these organoids, the researchers could tell which gene had been disabled by the CRISPR scissors, resulting in defective interneuron generation. They identified 13 such genes.
從那些iPS細胞,此些研究人員產生了大腦皮質下層的類器官。在培養皿中44天後,有些絲毫沒有顯現螢光。這顯示,它們未能產生中間神經元。藉由排序這些類器官的基因體,這些研究人員能斷言,哪個基因已經遭 CRISPR剪刀所失能,而導致產生有缺陷的中間神經元。他們確認了,13個此類基因。
To hunt for genes affecting interneuron migration, Pasca’s team placed subpallial organoids capable of generating interneurons beside healthy cortical organoids in numerous lab dishes.
為了尋找影響中間神經元遷移的基因。Pasca的團隊,於諸多實驗室培養皿中,將能產生中間神經元之大腦皮質下層的類器官,放在健康皮質的類器官旁邊。
The two types of organoids in each dish fused to form assembloids. After about 30 days of organoid cohabitation, the researchers pulled out about 1,000 assembloids from different dishes and cut each of them apart at the fusion junction. Then they compared the fluorescing interneurons in each assembloid’s subpallial section with those that had crossed the finish line into that assembloid’s cortical section.
於每一培養皿中,兩種類器官融合形成了組合體。在類器官共處大約30天後,此些研究人員從不同的培養皿中,取出大約1千個組合體,並在融合連接處,將其每一切隔開。之後,他們比較了,於每一組合體之大腦皮質下層部分中,發出螢光的中間神經元與那些已經越過終點線進入那組合體之皮質部分的中間神經元。
By sequencing the genomes of cells, the scientists could pinpoint genes in which a targeted deletion had impaired migration. They found 33 of them.
藉由排序細胞的基因體,此些科學家能確切找出一種,於其中有針對性之缺失,已經削弱遷移的基因。他們找到了其中的33個。
“The identification of 46 genes — close to 10% of all known neurodevelopmental-disorder-associated genes — whose dysfunction impairs interneuron development points to a subgroup of neurodevelopmental disorders that are characterized by inadequate inhibition of excitatory cortical neurons,” Pasca said.
Pasca宣稱:「46個接近所有已知,與神經發育障礙相關基因10%,其功能障礙損傷中間神經元發育之46個基因的確認,指向一子群,特徵是刺激性皮質神經元之抑制不足的神經發育障礙。」
Some of these genes have been previously uncharacterized, and their function unsuspected, Pasca said. But other genes have been identified in other studies. “This was a comfort factor,” Pasca said. “It meant we weren’t insane — this approach works.”
Pasca表示,其中有些基因先前未曾被描述,因此其功能未被懷疑過。不過,在其他研究中,已經確認了其他基因。Pasca宣稱:「這是一種慰藉因素,意味著我們不是瘋狂的。這種方法發生作用。」
網址:https://med.stanford.edu/news/all-news/2023/09/crispr-assembloids-neurodevelopmental.html
翻譯:許東榮
文章定位: