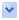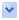圖1. 癌症藥物舒尼替尼(亮綠色)集中於某些亞細胞間隔室中,而不是均勻地分散於整個細胞中
Cancer drug sunitinib (bright green) concentrates in certain subcellular compartments rather than dispersing homogeneously throughout the cell.
Most drugs are small molecules that bind firmly to a specific target—some molecule in human cells that is involved in a disease—in order to work. For example, a cancer drug’s target might be a molecule that is abundant inside of cancer cells.
為了起作用,大多數藥物皆是,與特定標的(於人類細胞中,涉及疾病之某些分子)牢固結合的小分子。譬如,抗癌藥物的標的可能是一種,癌細胞內豐富的分子。
The drug should hypothetically travel freely throughout the cell until it comes to its target and then lock onto it, leading to a therapeutic action. However, small molecule drugs do not travel in such an unrestricted manner; instead, they tend to concentrate in specific regions of the cell. This is because each drug is capable of interacting with many more molecules than its target.
假設上,藥物應該於整個細胞中,自由移動直到抵達標的,然後鎖定它,從而產生治療效果。不過,小分子藥物並非以如此不受限制的方式移動。 相反地,它們傾向集中於細胞的特定區域。這是因為,每一藥物能與比其標的多得多的分子相互作用。
These other interactions tend to be weaker, like static cling versus the pull of a powerful magnet, but they can accumulate when molecules are concentrated together in cellular compartments called condensates. In these compartments, collective weak interactions may detain a significant percentage of drug molecules, keeping them localized either in the same neighborhood as their target or far away from it.
此些其他相互作用傾向較弱,如靜電吸附與強力磁鐵的拉力對比。不過,當分子集中於,被稱為縮合物的細胞間隔室時,它們會積聚。在這些間隔室中,集體的弱相互作用,會滯留很大比例的藥物分子。使得它們不是位於與其標的相同的區域,就是遠離標的。
Researchers in Whitehead Institute Member Richard Young’s lab are working to understand the chemical environments inside of different condensates and how these chemistries interact with those of small molecules.
於美國懷特海德研究所成員,Richard Young實驗室的研究人員們正在致力於,瞭解不同縮合物內部化學環境,及這些化學物質如何與那些小分子相互作用。
In research published in Nature Chemical Biology on September 28, Young and colleagues–including Regina Barzilay, the School of Engineering Distinguished Professor for AI and Health in the Massachusetts Institute of Technology (MIT) Computer Science & Artificial Intelligence Lab–trained a machine learning model to predict in which condensates a drug will concentrate based on their chemical features.
在2023年9月28日,發表於《自然•化學生物學》期刊的研究中,Young 及其同僚們(包括麻省理工學院(MIT)電腦科學暨人工智慧實驗室之人工智慧與健康工程學院的傑出教授Regina Barzilay)訓練了一種,根據縮合物之化學特色,來預測藥物會聚集於哪些縮合物的機器學習模型。
This work shows that interactions between condensates and small molecules help to determine where in the cell a small molecule will end up and what it will interact with, which may be relevant to understanding many cellular processes and to the design of safe and effective drugs.
該項研究顯示,縮合物與小分子之間的相互作用,有助於確定小分子會告終於細胞的何處,及其會與什麼相互作用。這會與瞭解諸多細胞變化過程,及安全且有效藥物的設計有重大關係。
If a large percentage of a small molecule drug, for instance, ends up in a condensate that does not contain the drug’s target, then much higher doses of the drug may be required for it to work, increasing the likelihood of toxicity and unintended side effects. Conversely, a drug designed to frequent the same condensate as its target would likely be more effective at lower—and so, typically, safer—doses.
譬如,倘若大比例的小分子藥物告終於,不具有藥物標的之縮合物中。那麼為了使其起作用,可能需要更高劑量的藥物,而增加毒性及非預期之副作用的可能性。相反地,針對經常與其標的相同之縮合物被設計的藥物,以較低(因此通常較安全)的劑量,可能會是更有效。
“Our work suggests that if you want to develop a very efficacious drug, then you should know where the target of the drug is in the cell with respect to these compartments,” says Young, who is also a professor of biology at MIT. “This would inform researchers and companies of the best way to develop a drug so that it is optimally concentrated near its target.”
也是麻省理工學院生物學教授的Young宣稱:「我們的研究暗示,倘若想開發一種非常有效的藥物,就上述間隔室而言,那麼應該知曉,於細胞中,何處是藥物的標的。」
Young lab researchers have spent years dedicated to the study of condensates, membrane-less cellular compartments that form when certain molecules tangle together to make a droplet within the cell, like a bead of oil suspended in water.
Young實驗室的研究人員們已經花數年時間,致力於縮合物的研究。當某些分子纏結在一起,於細胞內構成小滴時,形成的無膜細胞間隔室,如同懸浮於水中的一顆油珠。
These droplets function as organizational spaces in which the cell can gather together the right combination of molecules in the right location to perform their functions. Young and others have found evidence that condensates play this organizational role in many different cellular processes.
此些小滴起如同組織上的空間作用。在此些空間中,細胞能將分子的正確組合,聚集於正確的位置,以執行其作用。
They have also found evidence that drugs can concentrate in condensates, and that this may affect their efficacy. In 2020, Young and colleagues published a Science paper showing that the commonly used cancer drug cisplatin concentrates in transcriptional condensates, which keep the drug near the cancer-causing genes that it acts on.
他們也已經發現,藥物能集結於縮合物中的證據,而且這可能影響其功效。於2020年,Young及其同事們在《Science》期刊,發表了一篇論文,顯示普遍被使用的癌症藥物,順鉑(cisplatin)集結於轉錄的縮合物中,這使藥物保持於其作用的致癌基因附近。
Young lab postdoc Henry Kilgore and graduate student Kalon Overholt, co-first authors on the new paper, wondered what they would learn if they systematically tested whether and how different drugs concentrate in different condensates.
該篇新論文的共同首要撰文人,Young實驗室博士後的Henry Kilgore及研究生Kalon Overholt極想知曉,倘若他們系統性地測試不同藥物,是否及如何集中於不同縮合物中,他們會獲悉什麼。
First, they tested a large swathe of drugs to confirm that it is a common occurrence for drugs to concentrate in specific compartments rather than dispersing freely throughout the whole cell: they found that it is.
首先,他們測試了大量藥物以證實,藥物集結於特定間隔室中,而不是自由分散於整個細胞中,是一種普遍發生的情況:他們發現確實如此。
Next, they devised a system to study what might be causing drugs to concentrate in one condensate over another. They created models of three important types of condensates: one involved in gene transcription, one involved in gene repression, and the nucleolus—a large condensate inside of the nucleus that produces ribosomes.
接下來,他們設計了一個系統來研究,什麼可能導致藥物集結於一種縮合物中,而不是另一種縮合物中。他們創造了三種重要類型的縮合物模型:一種涉及基因轉錄,一種涉及基因抑制及核仁(細胞核內產生核醣體的大型縮合物)。
The researchers isolated the dominant type of protein that forms the framework of each of these three types of condensates, and formed simplified condensates made solely of each dominant protein.
此些研究人員分離了,形成這三種縮合物之每一框架的主要蛋白質類型,且形成了僅由每一種主要蛋白質組成的簡化縮合物。
Then the researchers assembled a library of more than 1500 small molecules with a wide variety of chemical features, and tested to see how strongly they would concentrate in each of the three model condensates.
然後,此些研究人員聚集了一個,多於1500種具有多類化學特色的小分子庫,並進行了測試以瞭解,它們能多強地集結於,上述三種模型縮合物的每一種中。
Most of the small molecules did favor one condensate over the others. Co-first author Peter Mikhael, a graduate student in Barzilay’s lab, trained a machine learning model on this data to identify patterns in how the small molecules sorted into different condensates.
大多數此些小分子確實偏好一種縮合物,超過其他縮合物。共同首要合撰人,Barzilay實驗室的研究生,Peter Mikhael根據此資料,訓練機器學習模型以識別,在此些小分子如何被分類成不同縮合物方面的模式。
The model found that the molecules that favored each type of condensate tended to have shared chemical features, and were more like each other than like molecules that favored other condensate types. It identified a number of features that seem to affect where molecules end up.
此模型發現,偏好每一類型縮合物的分子,傾向具有共有的化學特色,且與偏好其他縮合物類型的分子相較下,彼此更為相似。這確認了諸多,似乎影響分子告終於何處的特色。
For example, transcriptional condensates tended to attract small molecules containing electron-rich aromatic rings (a certain type of ring structure). Using these patterns, the model was very good at predicting in which of the simple condensates additional drugs would concentrate.
譬如,轉錄的縮合物傾向於吸引,具有富含電子芳香環(某類型的環結構)的小分子。利用此些模式,該模型非常擅長預測,更多之藥物會集結於哪些簡單的縮合物中。
Next, the researchers tested how well the model could predict where drugs would concentrate in live cells. It had moderate success. The lower accuracy reflects that the model was trained on simplified cases of single-protein condensates.
接下來,此些研究人員測試了,該模型能多適切預測,藥物會集結於活細胞中的何處。這獲得了差強人意的成功。較低的準確度反映出,該模型是在單一蛋白質之縮合物的簡化情況下,被訓練的。
In a cell, condensates contain hundreds of proteins, each of which may influence the local chemical environment, and condensates and other cellular compartments don’t exist in isolation: they compete to accumulate a drug.
在一個細胞中,縮合物具有數百種蛋白質,每一種蛋白質皆可能影響局部的化學環境,且縮合物及其他細胞間隔室,並非孤立存在的:它們競逐累積藥物。
The researchers are now working to understand the physical and chemical properties of these many proteins, so that they can improve their models. They also intend to narrow in on the specific mechanisms by which condensates create a favorable chemical environment for some molecules over others.
目前,此些研究人員正致力於,瞭解該諸多蛋白質的物理及化學屬性,以便能夠改善其模型。他們也打算縮小,縮合物為某些分子創造超越其他分子之有利化學環境的具體機制。
“In order for us to make use of condensate biochemistry, we would really like to have predictive power over where different molecules concentrate. While we’re still at the early stages, it’s exciting to envision a world where we have much finer control over where exactly drugs that we synthesize will go, such that they have maximum efficacy and minimal unwanted side-effects,” Mikhael says.
Mikhael宣稱:「為了使我們能利用縮合物的生物化學,我們真的想要具有預測不同分子集中於何處的能力。儘管我們仍處於初期階段,不過令人振奮的是,設想一個我們能更佳控制,我們合成之藥物確切會去何處的世界,從而使它們具有最大功效及最小不良副作用。」
In the meantime, the researchers hope that this work demonstrates the importance of re-thinking how cells are organized, and considering where molecules concentrate based on their chemical features.
在此同時,這些研究人員期盼,該項研究證明了,重新思考細胞如何組織,並根據其分子化學特色,考慮分子集結於何處的重要性。
"The inside of the cell has evolved to be highly compartmentalized, and that means the small molecules inside the cell are not distributed homogeneously," Overholt says. "It has been exciting to talk to experts from different fields and realize how many disciplines could potentially draw from our work on how molecules actually distribute in the cell.”
Overholt宣稱:「細胞內部已經演化成高度間隔化。因此那意味著,細胞內的小分子不是均勻地被分佈。與來自不同領域的專家們交談,而意識到有多少學科,潛在上可能從我們有關分子,在細胞中,實際如何分佈的研究推斷出,這一直是令人振奮的。」
The researchers anticipate that their work will be very useful to drug developers, but they also expect it to prove relevant to a number of other processes that occur within cells. More and more critical cellular processes are being found to rely on condensates to organize when and where relevant molecules concentrate.
此些研究人員期望,他們的研究對藥物開發人員們,會是非常有用。不過,他們也期待,這證明與細胞內發生的諸多其他變化過程,有所關聯。愈來愈多關鍵的細胞變化過程,被發現依賴縮合物,來組織相關分子何時、何處集結。
The better that researchers understand the chemical coding that regulates this organization, the better they will understand how essential cellular processes take place—and what may be going awry with them in disease.
研究人員們愈深入瞭解,調節該組織化的化學編碼,就愈能深入瞭解,至關重要之細胞變化過程如何發生,及它們在疾病中可能出什麼差錯。
“Everything we’ve learned about condensates in this study suggests that condensates and other cellular organelles have a powerful effect on the distribution of small molecules,” Kilgore says. “I'm convinced at this point that condensate small molecule selectivity has fundamental implications for biology and drug discovery.”
Kilgore宣稱:「在該項研究中,有關縮合物,我們獲悉的一切皆暗示,縮合物及細胞的其他細胞器,對小分子之分佈具有強大的影響。我確信了,在集結小分子之選擇性這一點,對生物學及藥物發現具有諸多主要的影響。」
網址:https://wi.mit.edu/news/machine-learning-helps-predict-drugs-favorite-subcellular-haunts
翻譯:許東榮
文章定位: