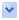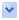圖1. 在阿茲海默氏症中,於大腦內嗅皮層中形成記憶的神經元(綠色)最先退化。 (圖援用自原文)
When Alzheimer’s disease strikes, the entire brain doesn’t crumble at once. Instead the mind unravels like grim clockwork, beginning with the telltale degradation of a group of brain cells in the entorhinal cortex. These so-called vulnerable neurons are responsible for shuttling experiences into memories. They are always the first to go.
當阿茲海默氏症侵襲時,整個大腦不會同時崩潰。而是頭腦,像堅韌的發條裝置,從內嗅皮層中,一群暴露內情的腦細胞退化,開始鬆散。此些所謂易受影響的神經元,負責將經歷穿梭移入記憶中。它們總是最早衰退。
Figuring out why patients lose these vulnerable neurons early on could be the key to discovering novel treatments for Alzheimer’s. Now, a new study sheds light on the inner workings of this subset of neurons and describes the molecular factors that render entorhinal brain cells uniquely sensitive to degeneration.
瞭解為何病患最先喪失,此些易受影響的神經元,或許是發現阿茲海默氏症新療法的關鍵。目前,一項新研究闡明了,此神經元子集隱晦、緩慢的進展,且發現了致使內嗅腦細胞,對退化特別敏感的諸多分子因素。
“If we can understand the peculiarities of the brain’s most vulnerable neurons, we can potentially open up new avenues for treatment,” says Jean-Pierre Roussarie, senior research associate in the late Paul Greengard’s Laboratory of Molecular and Cellular Neuroscience, who published the findings in Neuron.
於《神經元》期刊發表此些研究發現之已故(於 2019年4月13日逝世)美國生物醫學家,Paul Greengard的分子暨細胞神經科學實驗室資深研究夥伴,Jean-Pierre Roussarie宣稱:「倘若能瞭解大腦最易受影響之神經元的特殊性,則潛在上能開啟治療的新途徑。」
So far, attempts to develop a treatment for Alzheimer’s have largely failed. But most efforts undertaken have centered on the accumulation of Aβ peptides, which form plaques throughout the brain. These plaques are the first sign of Alzheimer’s, and the most studied.
到目前為止,諸多開發阿茲海默氏症療法的企圖,大部分已經失敗。不過,經從事的大多數嘗試,一向集中於一種,在整個大腦中,形成斑點之β肽的積聚上。此些斑點是阿茲海默氏症的最早徵兆,因此最受到研究。
The second sign of the disease is less celebrated, but may hold more promise. After the initial amyloid plaques form in the brain, a jumble of tau proteins known as neurofibrillary tangles clog the insides of neurons. Unlike amyloid plaques, this latter protein clump initially clusters solely within a distinct group of cells of the entorhinal cortex. The sheer predictability of the process makes it an attractive therapeutic target.
該種疾病的第二種徵兆較少受到關注,不過可能更具有發現療法的指望。在最初的澱粉樣蛋白斑點,於大腦中形成之後,一堆被通稱為神經原纖維纏結的雜亂tau蛋白,阻礙了神經元的本能。不像澱粉樣蛋白斑點,後者這種蛋白,起初僅在內嗅皮層的不同細胞群中,凝集成簇。此過程的十足可預測性,使其成為一種引人注目的治療標的。
But until now, scientists knew little about the nuances that make vulnerable neurons tick and tangle. With that in mind, the researchers set out to catalogue the genetic factors that render entorhinal neurons uniquely vulnerable to neurofibrillary tangles.
不過直到目前,科學家們鮮少知曉,有關使易受影響之神經元得以運作,及纏結的細微差異。考慮到這一點,此些研究人員著手進行編目分類了,致使內嗅神經元特別易受神經原纖維纏結影響的遺傳因素。
“There has been one trial after another, and we have accumulated a huge knowledge of the mechanisms that produce amyloid plaques,” Roussarie says. “But what is going on downstream of the amyloid accumulation, and how these plaques trigger neurofibrillary tangles within vulnerable neurons, is much more of a puzzle. It is a place where we could discover novel therapeutic targets.”
Roussarie宣稱:「一直有一次又一次的試驗,因此已經累積了大量,有關產生澱粉樣蛋白斑點的機制知識。不過,澱粉樣蛋白積聚的後階段發生了什麼事,及在易受影響的神經元中,此些斑點如何誘發神經原纖維纏結,更是一大謎團。這是一處,人們能發現新治療標的的處所。」
The biggest barrier to studying these brain cells was the absence of any easy way to distinguish vulnerable neurons from their neighbors. For Roussarie and his colleagues, BacTRAP provided an answer. Developed at Rockefeller by Greengard and Nathaniel Heintz, bacTRAP technology makes it possible to catalogue proteins within specific populations of neurons in mice.
研究這些腦細胞的最大障礙是,缺乏任何容易區分,易受影響之神經元與其鄰近神經元的方法。對Roussarie及其同僚們而言,細菌人工染色體-轉譯核醣體親和性的純化(bacTRAP:bacterial artificial chromosome ‐ translating ribosome affinity purification)技術,使得可能編目分類,於小鼠特定神經元種群中的蛋白質。
“We needed something like a microdissection of these neurons from the complex and mushy bowl of the brain,” says Marc Flajolet, acting head of the lab and coauthor on the study.
該項研究合撰人,上述實驗室代理負責人,Marc Flajolet宣稱:「他們需要某種,像此些來自複雜且糊狀碗大腦之神經元的顯微解剖。」
BacTRAP allowed the researchers to isolate the vulnerable neurons and analyze how they differ, genetically, from more resilient brain cells. A Princeton University team led by Olga Troyanskaya then designed computer algorithms to help the team focus upon only the genetic anomalies likely to be most relevant to neurodegeneration.
BacTRAP技術使此些研究人員,遺傳上得以從較能復原的腦細胞,分離出易受影響的神經元,並分析它們有多差異。之後,美國普林斯頓大學一支由Olga Troyanskaya領導的團隊,設計了諸多電腦演算法,來協助該團隊僅著重於,可能與神經退化最有關聯的遺傳異常。
“The goal was to form a big-picture view, rather than a list of genes,” Flajolet says. “Only by way of these sophisticated data-analysis frameworks can you get to the bottom of something as complicated as the neurodegenerative cascade in Alzheimer’s disease.”
Flajolet宣稱:「目標是形成一種大思維的概論,而不是一種基因列表。唯有經由這些複雜的數據分析框架,才能深入與阿茲海默氏症中,神經退化級聯反應一樣複雜的事情。」
The findings highlight a suite of genes that are likely involved in making entorhinal cortex neurons easy targets for degeneration.
此些研究發現強調了一組,就退化而言,可能涉及使內嗅皮質神經元輕易成為目標的基因。
The most compelling among them is thought to play a major role in the early stages of Alzheimer’s—deciding whether tau proteins clump into neurofibrillary tangles in the first place. The gene produces a protein called PTBP1, a so-called splice factor that directs cells to create one of two subtypes of tau protein.
其中最引人注目的是,被認為在阿茲海默氏症中,扮演一種重要角色。也就是,首先決定tau蛋白,是否凝聚成神經原纖維纏結。該基因產生一種,被稱為PTBP1的蛋白質。這是一種,指令細胞產生tau蛋白兩種亞型之一的所謂剪接因子。
Prior studies have shown that the characteristic protein clumps of Alzheimer’s occur specifically when the ratio of these two flavors of tau is disrupted—and the new findings suggest the disease might be driven by cells whose tau-variant levels are disturbed.
諸多先前的研究已經證實,阿茲海默氏症的特有蛋白凝塊,特別是在這兩種tau的比例,遭擾亂時發生。因此,這些新研究發現暗示,該種疾病可能是由,不同tau之含量遭擾亂的細胞所驅動。
“When tau popped out, there was a lot of excitement,” says Vicky Yao, an assistant professor in computer science at Rice University, and co-author of the Neuron report. “Once we figure out what makes neurons more vulnerable, that can lead to multiple avenues to decrease their vulnerability.”
該項於《神經元》期刊的報導合撰人,美國德州萊斯大學電腦科學方面的助理教授,Vicky Yao宣稱:「當tau突然出現時,有許多令人振奮之事。一旦人們瞭解了,使神經元更易受影響的原因,就能引領出多種降低其易受影響性的方法。」
Successful strategies for preventing and treating neurodegeneration will likely involve diverse approaches, adds Roussarie. Future drugs may need to target plaque formulation as well as neurofibrillary tangles, for example, and the first step toward preventing the latter will be to understand what makes some neurons prone to tangling in the first place.
Roussarie附言,預防及治療神經退化的成功對策,可能會涉及多種方法。譬如,除了神經原纖維纏結之外,未來的藥物也可能需要鎖定斑點的製劑。因此,邁向預防神經退化的第一步,首先應該是瞭解,什麼使得一些神經元易於纏結。
“The diversity of neurons was just not taken into account before,” Roussarie says. “A lot of people are studying neurofibrillary tangles, but only now are we beginning to address it through the prism of neuron vulnerability.”
Roussarie宣稱:「只是神經元的多樣性,先前未被納入考量。許多人正進行研究神經原纖維纏結,不過目前他們才開始,透過神經元易受影響性的折光物,來面對它。」
原文網址:https://www.rockefeller.edu/news/28453-why-memory-forming-neurons-vulnerable-to-alzheimers/
翻譯:許東榮
文章定位: